Technology
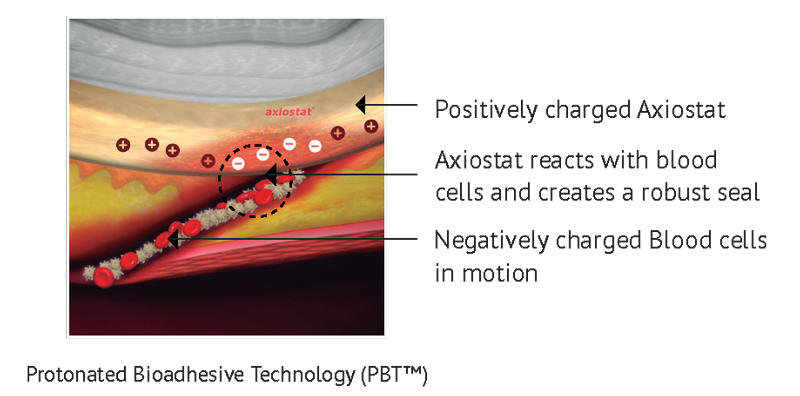
Protonated Bioadhesive Technology (PBT®)
Axio Biosolutions employs the proprietary Protonated Bioadhesive Polymer technology (PBT®) to make its biopolymer-based haemostats, drug delivery systems and scaffolds with tailorable bioadhesive properties. These preparations are unique in a sense that they adhere to the tissues when needed and easily detach when their job is done.
How it works
The essence of PBT® technology lies in maximizing the bioadhesive properties of native chitosan without chemical modifications in its backbone. Structurally, chitosan is a natural polysaccharide composed of monomers of glucosamine and N-acetyl glucosamine. Chitosan gets its cationic (+ve) charge through the protonation of primary amines ( NH 2 ) groups present in its glucosamine subunits. However, this protonation is pH-dependent, at a pH above 6.5 chitosan undergoes neutralization and loses the positive charge at the physiological pH of 7.4. Researchers have developed numerous chemical derivatives of chitosan to improve its cationic properties at physiological conditions. However, such chemical modifications often lead to other drawbacks such as inconsistency, increased processing costs, risk of toxicity, and reduction in chitosan’s molecular weight. Hence, we formulated chitosan with the (PBT®), which only relies on the physical properties and microscopic morphology of this natural material to maximize its cationic charge.
Indications
- Gunshot wounds
- Puncture or Stab wounds
- Blast injuries
- Cuts, Lacerations, Abrasions
- Arterial and Venous bleeding
- Neck and Head Trauma injuries
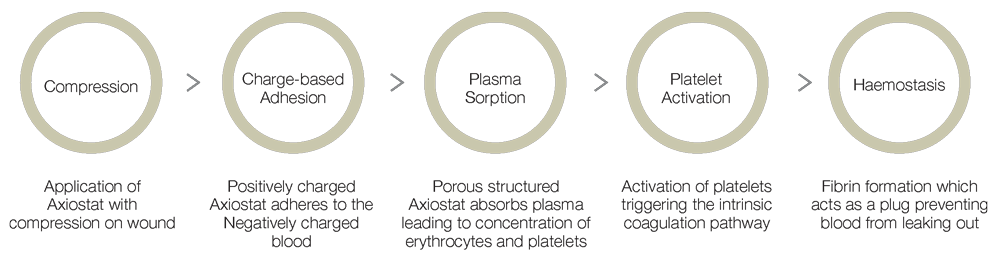
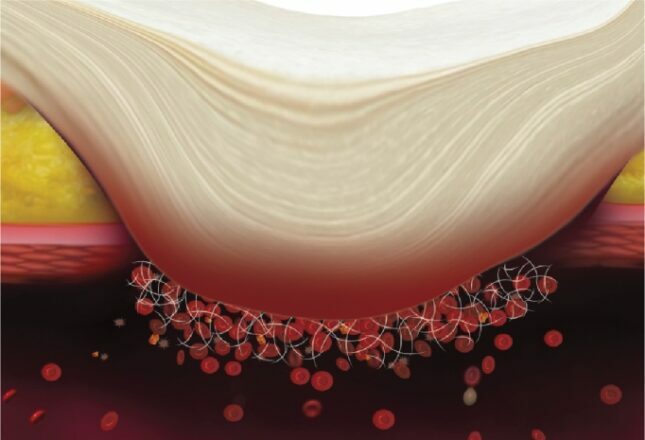
- Combined effect of bio-adhesion and strong blood clot formation induces instant haemostasis.
- Rapid blood plasma absorption and Entrapment of clotting factors and blood cells in the porous structure due to electrostatic interaction
- Maximization of inherent bio adhesion property of native chitosan without any chemical modification in chitosan backbone
- Creation of a robust seal at the combat injury site due to platelet activation and fibrin formation
- Faster haemostasis than natural blood clotting cascade
- Easy removal from combat injury site due to the neutralization at physiological pH

How it works
The essence of PBT® technology lies in maximizing the bioadhesive properties of native chitosan without chemical modifications in its backbone. Structurally, chitosan is a natural polysaccharide composed of monomers of glucosamine and N-acetyl glucosamine. Chitosan gets its cationic (+ve) charge through the protonation of primary amines ( NH 2 ) groups present in its glucosamine subunits. However, this protonation is pH-dependent, at a pH above 6.5 chitosan undergoes neutralization and loses the positive charge at the physiological pH of 7.4. Researchers have developed numerous chemical derivatives of chitosan to improve its cationic properties at physiological conditions. However, such chemical modifications often lead to other drawbacks such as inconsistency, increased processing costs, risk of toxicity, and reduction in chitosan’s molecular weight. Hence, we formulated chitosan with the (PBT®), which only relies on the physical properties and microscopic morphology of this natural material to maximize its cationic charge.
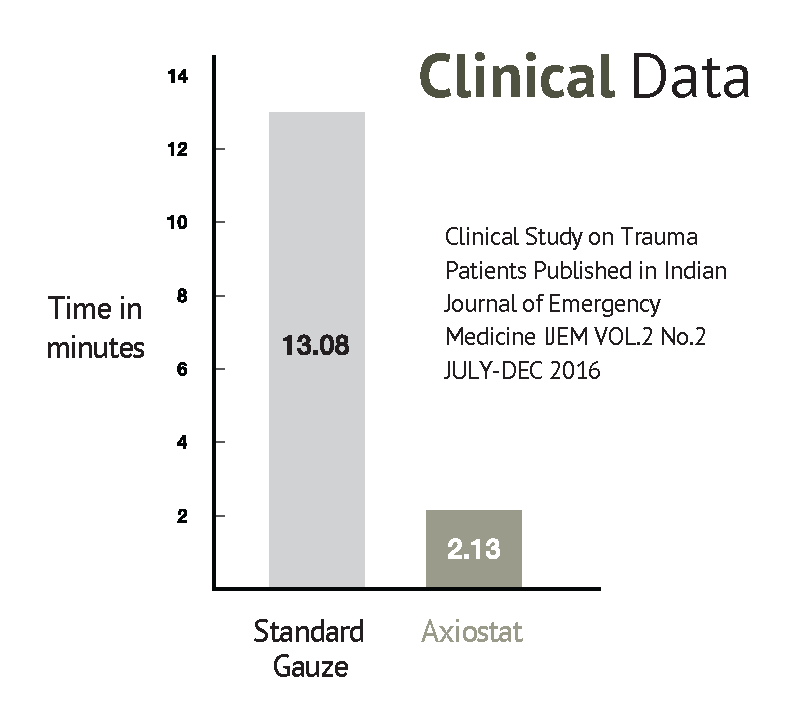
Haemostasis Process on application of Axiostat
Indications
- Deep injuries
- Gunshot injuries
- Head & Neck Trauma
- Cuts & Lacerations
- Lower extremities
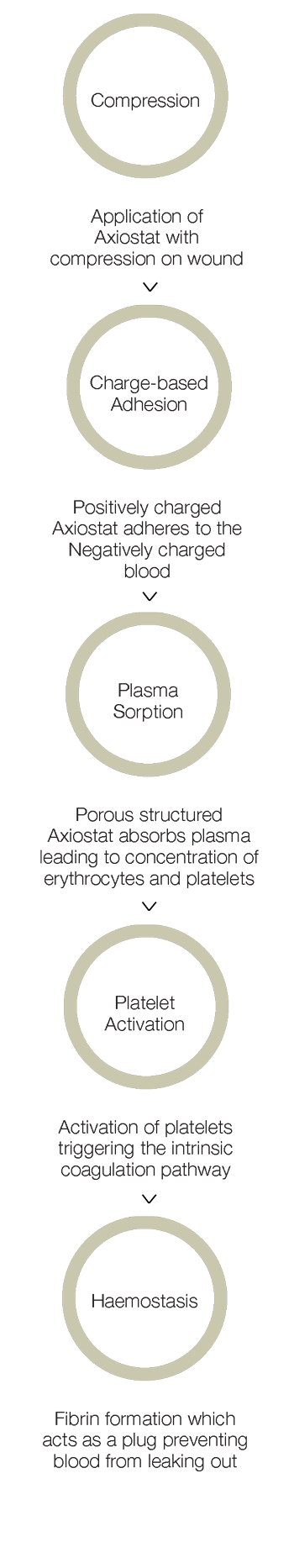
- Combined effect of bio-adhesion and strong blood clot formation induces instant haemostasis.
- Rapid blood plasma absorption and Entrapment of clotting factors and blood cells in the porous structure due to electrostatic interaction
- Maximization of inherent bio adhesion property of native chitosan without any chemical modification in chitosan backbone
- Creation of a robust seal at the combat injury site due to platelet activation and fibrin formation
- Faster haemostasis than natural blood clotting cascade
- Easy removal from combat injury site due to the neutralization at physiological pH





Our Key Regulatory Approvals/ Certifications

Scan QR to verify
CE Certificate - MaxioCel

Scan QR to verify
CE Certificate - Axiostat

Scan QR to verify
ISO 13485:2016

USFDA 510k Cleared
Axiostat Patch: 510k K202830
Axiostat Chitosan Haemostatic Dressing: 510k K172324
Serving
300+
Batallions Globally
Ideal for
IFAK, BFNA
& Bullet proof vests
30+
Annual Training Programs
Scientific Advisory Board

Dr Shiladitya Sengupta
Harvard Medical School, MIT

Dr S V Mahadevan
Stanford University Medical Center



 A collaborative study with Harvard
A collaborative study with Harvard